Search for a doctor or hospital in your network.

Search for a doctor or hospital in your network.

Get News & Updates Directly To Your Inbox
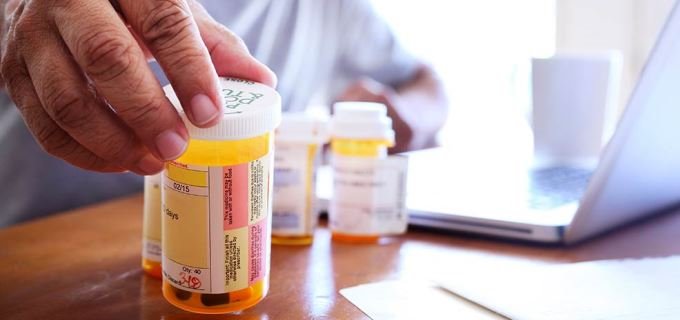
 Taking a generic drug is not like buying a store-brand soap. With soap, you might notice a difference. It may smell different. You may need to use more than the brand-name product. That is not true with generic drugs.
Taking a generic drug is not like buying a store-brand soap. With soap, you might notice a difference. It may smell different. You may need to use more than the brand-name product. That is not true with generic drugs. When companies make a brand-name drug, they get a patent. A patent stops other drug companies from making and selling the same drug before the patent runs out. When the patent on a brand-name drug expires, other drug companies can make a generic version. Generic versions can be equivalents or authorized generics, which are exact copies of the original brand name drug, or generic alternatives. Generic alternative drugs treat the same condition but do not have the same ingredients.
After getting approval from the U.S. Food and Drug Administration (FDA), ![]() the generic drug is made and sold under the active ingredient’s name.
the generic drug is made and sold under the active ingredient’s name.
| Myths | Facts |
| Generics are not as safe as brand-name drugs. | Generic equivalents use the same ingredients. They work the same. They have the same risk-benefit profile. |
| Generics are not as strong. | Generic drugs have the same quality, strength, purity and stability. |
| Generics take longer to act in the body. | The generic drug delivers the same amount of active ingredient in the same time span. |
| Brand-name drugs are made in modern manufacturing facilities, and generics are not. | Use of sub-standard facilities is not permitted by the FDA. |
| Brand-name drug | Generic drug |
| FDA Approved | FDA Approved |
| Marketed and developed by one drug company | Made and sold by more than one company |
| Sold under the trademark | Sold under the trademark (authorized generics) |
Generics don’t have to repeat the long clinical studies used to make the brand-name drug. The safety and value of the brand-name product has already been proven with research and many years of patient use.
Instead, companies that want to make a generic must show the FDA that it is a “bioequivalent” to the brand drug. That means:
The FDA considers several factors before it allows a company to make and sell a generic equivalent drug.
Now that you know the care and safety that goes into making generic drugs, you can take yours with peace of mind.
Blue Cross and Blue Shield of Illinois, a Division of Health Care Service Corporation,
a Mutual Legal Reserve Company, an Independent Licensee of the Blue Cross and Blue Shield Association
© Copyright 2026 Health Care Service Corporation. All Rights Reserved.
Verint is an operating division of Verint Americas, Inc., an independent company that provides and hosts an online community platform for blogging and access to social media for Blue Cross and Blue Shield of Illinois.
![]() File is in portable document format (PDF). To view this file, you may need to install a PDF reader program. Most PDF readers are a free download. One option is Adobe® Reader® which has a built-in screen reader. Other Adobe accessibility tools and information can be downloaded at https://www.adobe.com/trust/accessibility.html.
File is in portable document format (PDF). To view this file, you may need to install a PDF reader program. Most PDF readers are a free download. One option is Adobe® Reader® which has a built-in screen reader. Other Adobe accessibility tools and information can be downloaded at https://www.adobe.com/trust/accessibility.html. ![]()
![]() You are leaving this website/app ("site"). This new site may be offered by a vendor or an independent third party. The site may also contain non-Medicare related information. Some sites may require you to agree to their terms of use and privacy policy.
You are leaving this website/app ("site"). This new site may be offered by a vendor or an independent third party. The site may also contain non-Medicare related information. Some sites may require you to agree to their terms of use and privacy policy.
Powered by Verint